CLASS 10 Science
CHAPTER 4
CHAPTER 4
Carbon And Its Compounds (NCERT Notes)
Food, clothes, medicines, books, or many of the things are of carbon. In addition, all living structures are carbon based. The amount of carbon present in the earth’s crust and in the atmosphere is quite meagre. The earth’s crust has only 0.02% carbon in the form of minerals (like carbonates, hydrogen - carbonates, coal and petroleum) and the atmosphere has 0.03% of carbon dioxide. In spite of this small amount of carbon available in nature.
BONDING IN CARBON – THE COVALENT BOND
The reactivity of elements is explained as their tendency to attain a completely filled outer shell, that is, attain noble gas configuration. Elements forming ionic compounds achieve this by either gaining or losing electrons from the outermost shell. In the case of carbon, it has four electrons in its outermost shell and needs to gain or lose four electrons to attain noble gas configuration. If it were to gain or lose electrons –
(i) It could gain four electrons forming C4– anion. But it would be difficult for the nucleus with six protons to hold on to ten electrons, that is, four extra electrons.
(ii) It could lose four electrons forming C4+ cation. But it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons.
Carbon overcomes this problem by sharing its valence electrons with other atoms of carbon or with atoms of other elements. Not just carbon, but many other elements form molecules by sharing electrons in this manner. The shared electrons ‘belong’ to the outer shells of both the atoms and lead to both atoms attaining the noble gas configuration.
Hydrogen have the atomic number of hydrogen is 1. Hence hydrogen has one electron in its K shell and it requires one more electron to fill the K shell. So two hydrogen atoms share their electrons to form a molecule of hydrogen, H2. This allows each hydrogen atom to attain the electronic configuration of the nearest noble gas, helium, which has two electrons in its K shell. The shared pair of electrons is said to constitute a single bond between the two hydrogen atoms. A single bond is also represented by a line between the two atoms.
The atomic number of chlorine is 17. Chlorine forms a diatomic molecule, Cl2.
Oxygen can form a double bond between two oxygen atoms. This is because an atom of oxygen has six electrons in its L shell (the atomic number of oxygen is eight) and it requires two more electrons to complete its octet. So each atom of oxygen shares two electrons with another atom of oxygen . The two electrons contributed by each oxygen atom give rise to two shared pairs of electrons. This is said to constitute a double bond between the two atoms.
Nitrogen has the atomic number 7. In order to attain an octet, each nitrogen atom in a molecule of nitrogen contributes three electrons giving rise to three shared pairs of electrons. This is said to constitute a triple bond between the two atoms. The electron dot structure of N2 and its triple bond.
Methane is widely used as a fuel and is a major component of bio-gas and Compressed Natural Gas (CNG). It is also one of the simplest compounds formed by carbon. Methane has a formula CH4. Hydrogen has a valency of 1. Carbon is tetravalent because it has four valence electrons. In order to achieve noble gas configuration, carbon shares these electrons with four atoms of hydrogen.
Bonds which are formed by the sharing of an electron pair between two atoms are known as covalent bonds. Covalently bonded molecules are seen to have strong bonds within the molecule, but inter- molecular forces are small. This gives rise to the low melting and boiling points of these compounds. Since the electrons are shared between atoms and no charged particles are formed, such covalent compounds are generally poor conductors of electricity.
VERSATILE NATURE OF CARBON
The nature of the covalent bond enables carbon to form a large number of compounds. Two factors noticed in the case of carbon are –
(i) Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation. These compounds may have long chains of carbon, branched chains of carbon or even carbon atoms arranged in rings. In addition, carbon atoms may be linked by single, double or triple bonds. Compounds of carbon, which are linked by only single bonds between the carbon atoms are called saturated compounds. Compounds of carbon having double or triple bonds between their carbon atoms are called unsaturated compounds.
No other element exhibits the property of catenation to the extent seen in carbon compounds. Silicon forms compounds with hydrogen which have chains of upto seven or eight atoms, but these compounds are very reactive. The carbon-carbon bond is very strong and hence stable. This gives us the large number of compounds with many carbon atoms linked to each other.
(ii) Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other mono -valent element. Compounds of carbon are formed with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements giving rise to compounds with specific properties which depend on the elements other than carbon present in the molecule. Again the bonds that carbon forms with most other elements are very strong making these compounds exceptionally stable. One reason for the formation of strong bonds by carbon is its small size. This enables the nucleus to hold on to the shared pairs of electrons strongly. The bonds formed by elements having larger atoms are much weaker.
Allotropes of carbon
Allotropes or allotropism is the property of some chemical elements to exist in two or more different forms , in the same physical states ,known as allotropes of the element.
Diamond :In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure. Diamond is the hardest substance known while graphite is smooth and slippery.
Graphite: In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. One of these bonds is a double-bond, and thus the valency of carbon is satisfied. Graphite structure is formed by the hexagonal arrays being placed in layers one above the other. Graphite is also a very good conductor of electricity
These two different structures result in diamond and graphite having very different physical properties even though their chemical properties are the same. Diamonds can be synthesised by subjecting pure carbon to very high pressure and temperature. These synthetic diamonds are small but are otherwise indistinguishable from natural diamonds.
Fullerenes: form another class of carbon allotropes. The first one to be identified was C-60 which has carbon atoms arranged in the shape of a football. Since this looked like the geodesic dome designed by the US architect Buckminster Fuller, the molecule was named fullerene.
Saturated and Unsaturated Carbon Compounds
Saturated Carbon Compounds: An organic compound in which all the carbon atoms are connected by single bonds. These compounds are normally not very reactive. For example Methane, Ethane , Propane etc.
Unsaturated Carbon Compounds: An organic compound in which the carbon atoms are connected by double or by triple bonds. These compounds are very reactive. For example Methene, Ethene , Propene etc.
Chains, Branches and Rings
Carbon compounds methane, ethane and propane, containing respectively 1, 2 and 3 carbon atoms. Such ‘chains’ of carbon atoms can contain tens of carbon atoms.
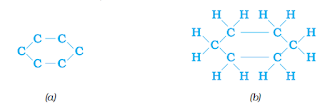
Straight and branched carbon chains, some compounds have carbon atoms arranged in the form of a ring. For example, cyclohexane has the formula C6H12 and the following structure –
Straight chain, branched chain and cyclic carbon compounds, all may be saturated or unsaturated. For example, benzene, C6H6 has the following structure –
All these carbon compounds which contain just carbon and hydrogen are called hydrocarbons. Among these, the saturated hydrocarbons are called alkanes. The unsaturated hydrocarbons which contain one or more double bonds are called alkenes. Those containing one or more triple bonds are called alkynes.
Functional groups
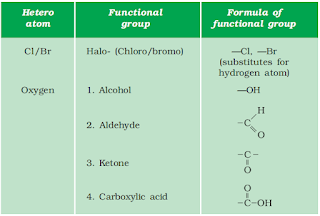
In a hydrocarbon chain, one or more hydrogens can be replaced by these elements, such that the valency of carbon remains satisfied. In such compounds, the element replacing hydrogen is referred to as a heteroatom. These heteroatoms confer specific properties to the compound, regardless of the length and nature of the carbon chain and hence are called functional groups. Free valency or valencies of the group are shown by the single line. The functional group is attached to the carbon chain through this valency by replacing one hydrogen atom or atoms.
Compounds in which the same functional group substitutes for hydrogen in a carbon chain is called a homologous series. For example, the chemical properties of CH3OH, C2H5OH, C3H7OH
and C4H9OH are all very similar. The general formula for alkenes can be written as CnH2n, where n = 2, 3, 4. As the molecular mass increases in any homologous series, a gradation in physical properties is seen. This is because the melting points and boiling points increase with increasing molecular mass. Other physical properties such as solubility in a particular solvent also show a similar gradation. But the chemical properties, which are determined solely by the functional group, remain similar in a homologous series.
Nomenclature of Carbon Compounds
The names of compounds in a homologous series are based on the name of the basic carbon chain modified by a “prefix” “phrase before” or “suffix” “phrase after” indicating the nature of the functional group. For example, the names of the alcohols are methanol, ethanol, propanol and butanol.
Naming a carbon compound can be done by the following method –
(i) Identify the number of carbon atoms in the compound. A compound having three carbon atoms would have the name propane.
(ii) In case a functional group is present, it is indicated in the name of the compound with either a prefix or a suffix .
(iii) If the name of the functional group is to be given as a suffix, the name of the carbon chain is modified by deleting the final ‘e’ and adding the appropriate suffix. For example, a three-carbon chain with a ketone group would be named in the following manner – Propane – ‘e’ = propan + ‘one’ = propanone.
(iv) If the carbon chain is unsaturated, then the final ‘ane’ in the name of the carbon chain is substituted by ‘ene’ or ‘yne’ . For example, a three-carbon chain with a double bond would be called propene and if it has a triple bond, it would be called propyne.
CHEMICAL PROPERTIES OF CARBON COMPOUNDS
Combustion
Combustion is a chemical process in which a substance reacts rapidly with oxygen and give off heat.
Carbon, in all its allotropic forms, burns in oxygen to give carbon dioxide along with the release of heat and light. Most carbon compounds also release a large amount of heat and light on burning. These are the oxidation reactions –
(i) C + O2 → CO2 + heat and light
(ii) CH4 + O2 → CO2 + H2O + heat and light
(iii) CH3CH2OH + O2 → CO2 + H2O + heat and light
Saturated hydrocarbons will generally give a clean flame while unsaturated carbon compounds will give a yellow flame with lots of black smoke. This results in a sooty deposit on the metal plate. However, limiting the supply of air results in incomplete combustion of even saturated hydrocarbons giving a sooty flame. The gas/kerosene stove used at home has inlets for air so that a sufficiently oxygen-rich mixture is burnt to give a clean blue flame. If you observe the bottoms of cooking vessels getting blackened, it means that the air holes are blocked and fuel is getting wasted. Fuels such as coal and petroleum have some amount of nitrogen and sulphur in them. Their combustion results in the formation of oxides of sulphur and nitrogen which are major pollutants in the environment.
Why do substances burn with or without a flame?
When candle or the LPG in the gas stove burns with a flame. However, you will observe the coal or charcoal in an ‘angithi’ sometimes just glows red and gives out heat without a flame. This is because a flame is only produced when gaseous substances burn. When wood or charcoal is ignited, the volatile substances present vapourise and burn with a flame in the beginning.
A luminous flame is seen when the atoms of the gaseous substance are heated and start to glow. The colour produced by each element is a characteristic property of that element. When you heat a copper wire in the flame of a gas stove and observe its colour. You have seen that incomplete combustion gives soot which is carbon.
Formation of coal and petroleum
Coal and petroleum have been formed from biomass which has been subjected to various biological and geological processes. Coal is the remains of trees, ferns, and other plants that lived millions of years ago. These were crushed into the earth, perhaps by earthquakes or volcanic eruptions. They were pressed down by layers of earth and rock. They slowly decayed into coal. Oil and gas are the remains of millions of tiny plants and animals that lived in the sea. When they died, their bodies sank to the sea bed and were covered by silt. Bacteria attacked the dead remains, turning them into oil and gas under the high pressures they were being subjected to. Meanwhile, the silt was slowly compressed into rock. The oil and gas seeped into the porous parts of the rock, and got trapped like water in a sponge.
Oxidation
Oxidation is the loss of electron or an increase in the oxidation state of an atom, an ions or of certain atoms in a molecule.
Carbon compounds can be easily oxidised on combustion. In addition to this complete oxidation, we have reactions in which alcohols are converted to carboxylic acids –
Some substances are capable of adding oxygen to others. These substances are known as oxidising agents. Alkaline potassium permanganate or acidified potassium dichromate are oxidising alcohols to acids, that is, adding oxygen to the starting material. Hence they are known as oxidising agents.
Addition Reaction
Addition Reaction is defined as an organic reaction where two or more molecules combine to form a larger one.
Unsaturated hydrocarbons add hydrogen in the presence of catalysts such as palladium or nickel to give saturated hydrocarbons. Catalysts are substances that cause a reaction to occur or proceed at a different rate without the reaction itself being affected.( Catalyst alter the rate of the reaction without making a change in the reaction ) This reaction is commonly used in the hydrogenation of vegetable oils using a nickel catalyst. Vegetable oils generally have long unsaturated carbon chains while animal fats have saturated carbon chains.
Animal fats generally contain saturated fatty acids which are said to be harmful for health. Oils containing unsaturated fatty acids should be chosen for cooking.
Substitution Reaction
A Substitution reaction ( also known as single displacement reaction or single substitution reaction) is a reaction during which one functional group in a chemical compound is replaced by another functional group. For example halogen show substitution reaction.
Saturated hydrocarbons are fairly unreactive and are inert in the presence of most reagents. However, in the presence of sunlight, chlorine is added to hydrocarbons in a very fast reaction. Chlorine can replace the hydrogen atoms one by one. A large number of products are usually formed with the higher homologues of alkanes.
CH4 + Cl2 → CH3Cl + HCl (in the presence of sunlight)
SOME IMPORTANT CARBON COMPOUNDS –
Properties of Ethanol
Ethanol is a liquid at room temperature . Its melting point is 156 K and boiling points 351 K . Ethanol is commonly called alcohol and is the active ingredient of all alcoholic drinks. It is a good solvent, it is also used in medicines such as tincture iodine, cough syrups, and many tonics. Ethanol is also soluble in water in all proportions. Consumption of small quantities of dilute ethanol causes drunkenness. However, intake of even a small quantity of pure ethanol (called absolute alcohol) can be lethal. Also, long-term consumption of alcohol leads to many health problems.
Reactions of Ethanol
(i) Reaction with sodium –
2Na + 2CH3CH2OH → 2CH3CH2O–
Na+ (Sodium ethoxide) + H2
Alcohols react with sodium leading to the evolution of hydrogen. With ethanol, the other product is sodium ethoxide.
(ii) Reaction to give unsaturated hydrocarbon: Heating ethanol at 443 K with excess concentrated sulphuric acid results in the dehydration of ethanol to give ethene –
The concentrated sulphuric acid can be regarded as a dehydrating agent which removes water from ethanol.
How do alcohols affect living beings?
When large quantities of ethanol are consumed, it tends to slow metabolic processes and to depress the central nervous system. This results in lack of coordination, mental confusion, drowsiness, lowering of the normal inhibitions, and finally stupour. The individual may feel relaxed but does not realise that his sense of judgement, sense of timing, and muscular coordination have been seriously impaired.
Unlike ethanol, intake of methanol in very small quantities can cause death. Methanol is oxidised to methanal in the liver. Methanal reacts rapidly with the components of cells. It causes the protoplasm to get coagulated, in much the same way an egg is coagulated by cooking. Methanol also affects the optic nerve, causing blindness.
Ethanol is an important industrial solvent. To prevent the misuse of ethanol produced for industrial use, it is made unfit for drinking by adding poisonous substances like methanol to it. Dyes are also added to colour the alcohol blue so that it can be identified easily. This is called denatured alcohol.
Alcohol as a fuel
Sugarcane plants are one of the most efficient convertors of sunlight into chemical energy. Sugarcane juice can be used to prepare molasses which is fermented to give alcohol (ethanol). Some countries now use alcohol as an additive in petrol since it is a cleaner fuel which gives rise to only carbon dioxide and water on burning in sufficient air (oxygen).
Properties of Ethanoic Acid
Ethanoic acid is commonly called acetic acid and belongs to a group of acids called carboxylic
acids. 5-8% solution of acetic acid in water is called vinegar and is used widely as a preservative
in pickles. The melting point of pure ethanoic acid is 290 K and hence it often freezes during winter
in cold climates. This gave rise to its name glacial acetic acid.
The group of organic compounds called carboxylic acids are obviously characterised by a special acidity. However, unlike mineral acids like HCl, which are completely ionised, carboxylic
acids are weak acids.
Reactions of ethanoic acid:
(i) Esterification reaction: Esters are most commonly formed by reaction of an acid and an alcohol.
Ethanoic acid reacts with absolute ethanol in the presence of an acid catalyst to give an ester –
(ii) Reaction with a base: Like mineral acids, ethanoic acid reacts with a base such as sodium hydroxide to give a salt (sodium ethanoate or commonly called sodium acetate) and water:
NaOH + CH3COOH → CH3COONa + H2O
(iii) Reaction with carbonates and hydrogencarbonates: Ethanoic acid reacts with carbonates and hydrogencarbonates to give rise to a salt, carbon dioxide and water. The salt produced is commonly called sodium acetate.
SOAPS AND DETERGENTS
The effect of soap in cleaning
Most dirt is oily in nature and oil does not dissolve in water. The molecules of soap are sodium or potassium salts of long-chain carboxylic acids. The ionic-end of soap dissolves in water while the carbon chain dissolves in oil. The soap molecules, thus form structures called micelles where one end of the molecules is towards the oil droplet while the ionic-end faces outside. This forms an emulsion in water. The soap micelle thus helps in dissolving the dirt in water and we can wash our clothes clean.
Micelles
Soaps are molecules in which the two ends have differing properties, one is hydrophilic, that is, it dissolves in water, while the other end is hydrophobic, that is, it dissolves in hydrocarbons. When soap is at the surface of water, the hydrophobic ‘tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water. Inside water, these molecules have a unique orientation that keeps the hydrocarbon portion out of the water. This is achieved by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called a micelle. Soap in the form of a micelle is able to clean, since the oily dirt will be collected in the centre of the micelle. The micelles stay in solution as a colloid and will not come together to precipitate because of ion-ion repulsion. Thus, the dirt suspended in the micelles is also easily rinsed away. The soap micelles are large enough to scatter light. Hence a soap solution appears cloudy.
(From NCERT Book)