CLASS 10 Science
CHAPTER 1
Chemical Reactions And Equations (NCERT Notes)
CHEMICAL EQUATIONS
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae. It comprises of reactant and product. The reactants are written on the left-hand side (LHS) with a plus sign (+) between them. And products are written on the right-hand side (RHS) with a plus sign (+) between them. The arrowhead points towards the products, and shows the direction of the reaction. The word-equation for the reaction would be –
Magnesium + Oxygen (Reactants) → Magnesium oxide (Product)
Writing a Chemical Equation
Chemical equations can be made more concise and useful if we use chemical formulae instead of words. A chemical equation represents a chemical reaction. For example formulae of magnesium, oxygen and magnesium oxide, equation can be written as –
Mg + O2 → MgO
Count and compare the number of atoms of each element on the LHS and RHS of the arrow. Is the number of atoms of each element the same on both the sides? If not, then the equation is unbalanced because the mass is not the same on both sides of the equation.
Balanced Chemical Equations
According to the law of conservation of mass : Mass can neither be created nor destroyed in a chemical reaction. That is, the total mass of the elements present in the products of a chemical reaction has to be equal to the total mass of the elements present in the reactants.
The number of atoms of each element remains the same, before and after a chemical reaction.
The word-equation for the reaction can be represented as –
Zinc + Sulphuric acid → Zinc sulphate + Hydrogen
The word-equation may be represented as –
Zn + H2SO4 → ZnSO4 + H2
As the number of atoms of each element is the same on both sides of the arrow, Equation is a balanced chemical equation.
Let us try to balance the following chemical equation –
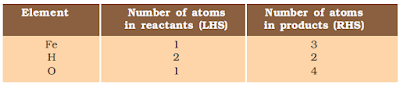
Fe + H2O → Fe3O4 + H2
Step I: To balance a chemical equation, first draw boxes around each formula. Do not change anything inside the boxes while balancing the equation.
Step II: List the number of atoms of different elements present in the unbalanced equation .
the LHS.
To balance the oxygen atoms –
Step IV: Fe and H atoms are still not balanced. Pick any of these elements to proceed further. Let us balance hydrogen atoms in the partly balanced equation.
To equalise the number of H atoms, make the number of molecules of hydrogen as four on the RHS.
The equation would be –
Step V: Examine the above equation and pick up the third element which is not balanced. You find that only one element is left to be balanced, that is, iron.
Step VI: Finally, to check the correctness of the balanced equation, we count atoms of each element on both sides of the equation.
3Fe + 4H2O → Fe3O4 + 4H2 (balanced equation)
The numbers of atoms of elements on both sides of Equation are equal. This equation is now balanced. This method of balancing chemical equations is called hit-and-trial method as we make trials to balance the equation by using the smallest whole number coefficient.
Step VII: Writing Symbols of Physical States :To make a chemical equation more informative, the physical states
of the reactants and products are mentioned along with their chemical formulae. The gaseous, liquid, aqueous and solid states of reactants and products are represented by the notations (g), (l), (aq) and (s), respectively. The word aqueous (aq) is written if the reactant or product is present as a solution in water.
The balanced Equation becomes -
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
Note that the symbol (g) is used with H2O to indicate that in this reaction water is used in the form of steam.
Usually physical states are not included in a chemical equation unless it is necessary to specify them.
Sometimes the reaction conditions, such as temperature, pressure, catalyst, etc., for the reaction are indicated above and/or below the arrow in the equation. For example –
TYPES OF CHEMICAL REACTIONS
Chemical reactions involve the breaking and making of bonds between atoms to produce new substances.
Combination Reaction
When two or more substances (elements or compounds) combine to form a single product, the reactions
are called combination reactions. For example Calcium oxide reacts vigorously with water to produce slaked lime (calcium hydroxide) releasing a large amount of heat.
CaO(s) (Quick lime) + H2O(l) → Ca(OH)2(aq)(Slaked lime)
Calcium hydroxide reacts slowly with the carbon dioxide in air to form a thin layer of calcium carbonate on the walls. Calcium carbonate is formed after two to three days of white washing and gives a shiny finish to the walls. It is interesting to note that the chemical formula for marble is also CaCO3.
Ca(OH)2(aq) (Calcium hydroxide) + CO2(g) → CaCO3(s) (Calcium carbonate) + H2O(l)
Examples of combination reactions.
(i) Burning of coal
C(s) + O2(g) → CO2(g)
(ii) Formation of water from H2(g) and O2(g)
2H2(g) + O2(g) → 2H2O(l)
Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. Examples of exothermic reactions are –
(i) Burning of natural gas
CH4(g) + 2O2 (g) → CO2 (g) + 2H2O (g)
(ii) Do you know that respiration is an exothermic process?
During digestion, food is broken down into simpler substances. For example, rice, potatoes and bread contain carbohydrates. These carbohydrates are broken down to form glucose. This glucose combines with oxygen in the cells of our body and provides energy. This overall reaction is respiration.
C6H12O6(aq) + 6O2(aq) → 6CO2(aq) + 6H2O(l) + energy (Glucose)
(iii) The decomposition of vegetable matter into compost is also an example of an exothermic reaction.
Decomposition Reaction
Reaction in which a single reactant breaks down to give simpler products. This is called a decomposition reaction.
For example : Ferrous sulphate crystals (FeSO4, 7H2O) lose water when heated and the colour of the crystals changes. It then decomposes to ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3). Ferric oxide is a solid, while SO2 and SO3 are gases.
Decomposition of calcium carbonate to calcium oxide and carbon dioxide on heating is an important decomposition reaction used in various industries. Calcium oxide is called lime or quick lime. It has many uses – one is in the manufacture of cement.
When a decomposition reaction is carried out by heating, it is called thermal decomposition.
CaCO3(s) (Limestone) + Heat → CaO(s) + CO2(g) (Quick lime)
Another example of a thermal decomposition reaction
2Pb(NO3)2(s) (Lead nitrate) + Heat → 2PbO(s) (Lead oxide) + 4NO2(g) (Nitrogen dioxide) + O2(g) (Oxygen)
White silver chloride turns grey in sunlight. This is due to the decomposition of silver chloride into silver and chlorine by light.
2AgCl(s) + Sunlight → 2Ag(s) + Cl2(g)
Silver bromide also behaves in the same way.
2AgBr(s) + Sunlight → 2Ag(s) + Br2(g)
This reactions are used in black and white photography.
The decomposition reactions require energy either in the form of heat, light or electricity for breaking down the reactants.
Reactions in which energy is absorbed are known as endothermic reactions.
Displacement Reaction
Displacement Reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. Both metal and non -metal takes part in displacement reaction.
The iron nail become brownish in colour and the blue colour of copper sulphate solution fade off.
Fe(s) + CuSO4(aq) (Copper sulphate) → FeSO4(aq) (Iron sulphate) + Cu(s)
Other examples of displacement reactions are
Zn(s) + CuSO4(aq) (Copper sulphate) → ZnSO4(aq) (Zinc sulphate) + Cu(s)
Pb(s) + CuCl2(aq) (Copper chloride) → PbCl2(aq) (Lead chloride) + Cu(s)
Zinc and lead are more reactive elements than copper. They displace copper from its compounds.
Double Displacement Reaction
Reactions in which there is an exchange of ions between the reactants are called double displacement reactions.
Any reaction that produces a precipitate can be called a precipitation reaction.
Na2SO4(aq) (Sodium sulphate) + BaCl2(aq) (Barium chloride) → BaSO4(s) (Barium sulphate) + 2NaCl(aq) (Sodium chloride)
What causes this? The white precipitate of BaSO4 is formed by the reaction of SO-24 and Ba2+. The other product formed is sodium chloride which remains in the solution. Such reactions in which there is an exchange of ions between the reactants.
Oxidation and Reduction
If a substance gains oxygen during a reaction, it is said to be oxidised. If a substance loses oxygen during a reaction, it is said to be reduced.
The surface of copper powder becomes coated with black copper(II) oxide. Why has this black
substance formed? This is because oxygen is added to copper and copper oxide is formed.
2Cu + O2 Heat →2CuO
If hydrogen gas is passed over this heated material (CuO), the black coating on the surface turns brown as the reverse reaction takes place and copper is obtained.
CuO +H2 Heat → Cu + H2O
The copper(II) oxide is losing oxygen and is being reduced. The hydrogen is gaining oxygen and is being oxidised.
The reaction in which one reactant gets oxidised while the other gets reduced during a reaction. Such reactions are called oxidation-reduction reactions or redox reactions.
If a substance gains oxygen or loses hydrogen during a reaction, it is oxidised. If a substance loses oxygen or gains hydrogen during a reaction, it is reduced.
When a magnesium ribbon burns with a dazzling flame in air (oxygen) and changes into a white substance, magnesium oxide.
THE EFFECTS OF OXIDATION REACTIONS IN EVERY DAY LIFE
Corrosion
When a metal is attacked by substances around it such as moisture, acids, etc., it is said to corrode and this process is called corrosion. The black coating on silver and the green coating on copper are other examples of corrosion. Iron articles are shiny when new, but get coated with a reddish brown powder when left for some time. This process is commonly known as rusting of iron.
Corrosion causes damage to car bodies, bridges, iron railings, ships and to all objects made of metals, specially those of iron. Corrosion of iron is a serious problem.
Corrosion can be prevented by painting , electro painting, greacing . oiling ,by making it stainless steel.
Rancidity
Rancidity is a condition produced by arial oxidation of fat unsaturated fat present in food and other product, marked by unpleasant odour or flavour.
When fats and oils are oxidised, they become rancid and their smell and taste change. Usually substances which prevent oxidation (antioxidants) are added to foods containing fats and oil. Keeping food in air tight containers helps to slow down oxidation. Do you know that chips manufacturers usually flush bags of chips with gas such as nitrogen to prevent the chips from getting oxidised ?
( From NCERT Book )